Originally published by WIRED
WIRED Features Island Conservation Project Director Karl Campbell

WIRED features GBIRd and Island Conservation Project Director Karl Campbell in an article about the potential for gene drives to help prevent extinctions of island species.
KARL CAMPBELL IS a craftsman bedeviled by bad tools. He’s a middle-aged, medium-size, muscular Australian with a five-day beard and an intense gaze who seems perpetually coiled, even angry, when at rest. He’s smiling and relaxed only when his body is in motion—preferably fixing something, building something, or killing something.
His craft—and his mission—is saving as many endangered species as he can, in what he reckons the most effective way. It’s a grueling job by which he creates life out of death, preventing the catastrophe of irreversible extinction with a tide of blood. He kills goats and rats and other human-introduced animals that threaten rare island creatures, but his tools—traps, long-range rifles, and poisons—are brutal, deployable only on a small scale and wildly indiscriminate. To excise the rat, say, from an ecosystem requires a sledgehammer that falls on many species.
Ecology is complex, even on tiny islands, and things don’t always go according to plan. In 2012, for instance, Campbell, who works for an organization called Island Conservation, helped round up the 60 Galapagos hawks that lived on Pinzón Island, a steep volcanic nubbin in the Galapagos chain, so they wouldn’t eat the rats that Campbell was about to poison. But when the rare raptors were released back into the wild after a couple of weeks, they began dropping like flies. It turned out the poison was lurking in lava lizards—hawk prey.
Campbell is now preparing for an even riskier maneuver: using a fiercely potent poison for the complete obliteration of rats on a 70-square-mile Galapagos island called Floreana. The island was once home to a chocolate-brown bird with a perky tail called the Floreana mockingbird, but the rats eat its eggs and chicks, so the bird remains on only a couple of islets. Once the rats are gone, the mockingbird could be brought back to the place for which it was named. The rats’ destruction will be brought about by a carpet-bombing of lethal pellets: Some 300 tons of poisoned cereal will be dumped from helicopters, enough to kill every rat on the island. The problem is that 150 people and their farm animals also live on Floreana.
On a cool and sunny Monday last August, Campbell and I hopped in a local farmer’s battered Toyota Land Cruiser and headed for the highlands of Floreana. Rats are no friends to farmers either, and Campbell pointed to some corn in Claudio Cruz’s fields that had been nibbled away by sharp rodent teeth. Cruz had stacked two bright-red shipping containers up on blocks—one a gift from Island Conservation, one he bought himself. They will be used to store uncontaminated animal feed when the poison comes, tentatively in 2020. Island Conservation will also build coops, sties, and stables for the island’s chickens, pigs, and horses. It will buy sentinel pigs that will live outside the sties and be slaughtered at intervals so their livers can be tested for poison. The other pigs won’t be able to emerge until the sentinel pigs’ livers are clear. This might take three years. Parents will have to keep close watch over small children lest they eat pellets off the ground. Scores of native animals—likely including finches and short-eared owls—will be captured and held in aviaries both on and off the island. Campbell expects it will take 10 years and $26 million to clear this small island of rats.
All this is why Campbell has begun pushing for research into a much more precise and effective tool—one you might not associate with nature-loving conservationists. Self-perpetuating synthetic genetic machines called gene drives could someday alter not just one gene or one rat or even a population of rats but an entire species—of rats, mosquitoes, ticks, or any creature. And this biological technology promises to eliminate these destructive animals without shedding a drop of blood. So Campbell has spent the past few years dividing his time between old-fashioned killing and traveling the world to pitch the gene drive approach to ecologists, ethicists, and prospective donors. He’s not alone in his enthusiasm. Institutions from the US military’s research agency to the Gates Foundation to the government of New Zealand are looking to gene drives as possible solutions for big problems (malaria, Lyme disease, species extinction). But the methods also contain the threat of unleashing another problem: They could change species, populations, and ecosystems in unintended and unstoppable ways.

When Linda Cayot, project coordinator for a Galapagos-based restoration program called Project Isabela, picked Campbell for an internship with the organization back in the late 1990s, she recalls that one of his virtues was a “certain macho army roughness.” Campbell had learned to shoot firearms and repair vehicles in the Australian Army Reserve. He’d spent a few weeks volunteering to catch and arrest antelope poachers in Malawi. He was well suited to the demands of the work on the islands: Once he slashed open his thumb and had a friend stitch it up in the field; another time he came back from a visit to a remote volcano with most of the skin on his feet peeling off. He didn’t bother to mention it.
Perhaps because of his disdain for comfort, Campbell thrived in the harsh volcanic landscape of the Galapagos, with its strange and wonderful wildlife. Because humans, with their talent for destruction, found these volcanic islands so late in history, 95 percent of the original and unique species remain. There are giant tortoises, marine iguanas that shoot salt snot from their nostrils, and waved albatrosses that glide on 8-foot-wide wings, eyes like black tapioca balls.
When humans did establish permanent residency on the islands, starting in 1805, they brought beasts of burden, animals for meat, and the clever and voracious rat, hidden in the holds of their ships. The animals of the Galapagos, like island species everywhere, had let down their defenses over evolutionary time and simply could not cope with these bulldozing newcomers. Some had lost their ability to fly away; some had taken up nesting on the ground, with their eggs out in the open; perhaps most dangerously, they had lost their fear. Even when invaders didn’t eat the native fauna, they did damage in other ways. On the Galapagos, goats ate so many plants that one estimate claimed that 60 percent of the Galapagos’ 194 endemic plants were threatened with extinction—not to mention the islands’ giant tortoises, which were starving to death with no plants to eat.
For Project Isabela, Campbell shot goats with semiautomatic rifles, mostly from helicopters, occasionally on foot with dogs. But he quickly recognized the imperfection of these methods. He came up with a strategy for inducing sexual receptivity in females in order to lure other goats out of hiding, round them up, and shoot them. The resulting “Mata Hari” goats were a big success and propelled Campbell to a kind of fame, but he dismisses the technique as a mere “incremental innovation.” He was looking for a “transformative innovation.”
In 2006 Campbell went to work for Island Conservation, taking his skills beyond the Galapagos. He has helped rid San Nicolas Island, in California, of feral cats; Choros Island, Chile, of rabbits; and Desecheo Island, Puerto Rico, of rhesus macaques. But every eradication is a grind, and Campbell is vexed by the scale of the problem: There are 465,000 islands on Earth, home to 41 percent of endangered land vertebrates, and most of the islands with endangered species also have introduced species on them. “We are barely scratching the surface,” Campbell says.
Then, in 2011, Campbell stumbled upon an idea that smelled like the transformative innovation he had been looking for.
An entomologist at North Carolina State University named Fred Gould had written a paper positing that genetic engineering techniques that had been used with insects were ripe for deployment in other troublesome species like rodents. (Along with driving island species extinct, rats and mice eat enough rice each year to feed 180 million people, and they transmit Lyme disease and hantavirus.) Scientists could use genetic engineering to favor certain traits, Gould pointed out, and push them through wild populations. Normally, for any given gene that comes in different types, an offspring has a 50 percent chance of inheriting the mother’s version and a 50 percent chance of inheriting the father’s version. But some genes have naturally evolved a way to cheat this system—if one parent has the gene an offspring has a virtually 100 percent chance of inheriting that version. That mysterious cheat code is called a gene drive, and if scientists could engineer a synthetic gene drive, they could spread a desired trait through a population and down through generations. To eradicate rats on an island, you might push a gene for infertility that would cause a population to crash once it reached a certain prevalence—no poisons necessary. The rodents would simply fade away, like heirless lords.
Campbell invited himself for a visit to Gould’s lab in Raleigh. As you do, Gould turned to the internet to figure out who Campbell was. “I was just shocked,” Gould says. “If you look at the Island Conservation website it is all woodsy-greensy.” A lot of passionate environmentalists are opposed to genetic engineering. Gould asked Campbell, “Do you know what you are getting into?”
Campbell did. But he didn’t care that other conservationists considered genetic engineering too risky to attempt and too unnatural to countenance. He wanted to stop extinctions. Gould liked the man’s pragmatism.
Gould’s ideas were theoretical. But in 2012 the prospect of making the theoretical real suddenly got a lot better with the discovery of the Crispr technique, a new way to edit genes quickly, cheaply, and precisely. With Crispr, any DNA sequence could be precisely cut and pasted into any location in any genome.

About two years later, Kevin Esvelt, a geneticist then at Harvard University, put gene drives and Crispr together. Instead of poking a big fat glass needle loaded up with synthetic DNA into every organism that you want to change, you do it once, with a gene drive that encodes not only the gene you want (or the deactivation of the gene you don’t want) but also instructions to do that same manipulation with the Crispr technique in another genome. So when your altered organism mates, its chromosome gets to work, engineering the chromosome inherited from the mate too. This guarantees that the offspring has the desired change, plus the instructions to make the desired change.
When the offspring reaches maturity and mates, the process repeats. In a perfect “global” gene drive, 100 percent of offspring have the gene drive carrying the desired trait.
The possibility was a tantalizing one for conservation. You could start thinking way bigger than Floreana: the Galapagos island of Santa Cruz, with its 12,000 people. Or, hell, Australia—Campbell’s home country, a massive island with dozens of species endangered largely because of introduced cats and foxes. You could fix every island in the world.
The idea of using gene drives to save species began to hum. Campbell helped organize people from Island Conservation and researchers in the United States, Australia, and New Zealand, as well as the United States Department of Agriculture, to research the approach. The group formalized as the Genetic Biocontrol of Invasive Rodents program, or GBIRd. In June 2016, Paul Thomas, a mouse geneticist from the University of Adelaide, Australia, visited Gould in North Carolina and got fired up. Thomas felt that his lab could be the place to figure out how to make a synthetic gene drive work in rodents. If he could succeed in lab mice, he could succeed with the wild mice and rats that eat the eggs and young of rare species on islands. Thomas joined GBIRd.
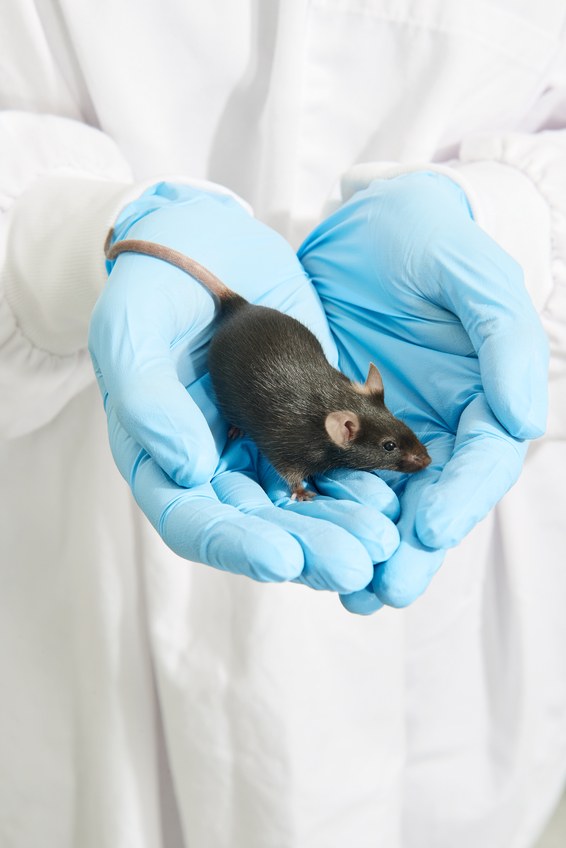
When I visited Paul Thomas’ lab in Adelaide in August, I accompanied a grad student named Chandran Pfitzner to the mouse rooms. Before entering, we put on blue suits, hair nets, and masks. Pfitzner sprayed down my notebook with antiseptic and led me down a warm, hushed hallway to a room full of plexiglass mouse boxes on racks. The rooms were surprisingly quiet, almost muffled, with the merest undertone of animals burrowing and gnawing. The research mice were tiny and smelled like sweet sawdust and salt. Pfitzner, consulting his notes on the cracked screen of his phone, plucked one up by the tail, grabbed a tiny hole punch, and awkwardly excised a tiny circle of skin out of its ear. The mouse didn’t make a sound.
This mouse was created in another building on campus. There, a fertilized egg was pierced with a glass needle and injected with the necessary ingredients for overriding the random chance of inheritance: the molecular “scissors” used in Crispr engineering, a guiding molecule that tells it where to cut, and a promoter to activate the scissors in the right tissues (see “How to Kill Off a Species, Nicely”). In this case, the Crispr-snipped gene was not for infertility but for coat color. The idea was to make the synthetic gene drive work first for a trait for which it is easy to check the results of at a glance. If the drive was working, the mouse would be albino. Instead, it was a rather lovely taupe. Pfitzner put the mouse back in the box.
After we left the mouse room and stripped off our protective gear, Pfitzner popped the little piece of ear skin under a microscope. He wanted to see if the elements of the gene drive were in place. The scientists also had inserted fluorescent proteins next to the “scissors” and other components, and the mouse flesh glowed with two colors, maraschino-cherry red and a neon green, under an inverted fluorescence microscope. All the pieces were there, but the taupe coat was proof that the elements weren’t functioning.
Out of 30 mice, Thomas and Pfitzner did get three dark-gray mice with patches and sprays of white, suggesting that the drive worked in some, but not all, of their cells. “It is early days,” Thomas said, gazing rather forlornly at a picture of a mosaic mouse that he printed out for me. Science is a long haul, but Thomas has no doubt his team will crack the code. It’s simply a matter of time. He expects the coat-color gene drive to function in the lab by about 2020, and one that could cause infertility shortly thereafter.
Thomas and some colleagues in applied math modeled how long it would take to eradicate an island mouse population of 50,000 by introducing just 100 mice engineered with an infertility gene drive. The answer was less than five years.
In the tiny ear-punched mouse, then, was the seed of an unprecedented possibility—that humans could not just change a few mice in an Australian lab but permanently alter all mice, everywhere. The 30-gram wriggler portends a kind of power over nature we’ve never had before: an ability to edit—or to delete—whole species.
This potential means that Thomas is taking special precautions. He understands that it could be perilous to the environment—and would certainly be perilous for public relations—should a mouse with a drive toward albinism or infertility escape its plexiglass box and start mating with the free mouse population. So the first thing he did was create a dedicated line of mice for these experiments. Thomas’ gene drive will only activate in the presence of a unique chunk of bacterial DNA that was engineered into the hole-punched mouse and its companions. That way, if one of these little mice slips out into the hills around Adelaide and mates with a house mouse, the gene drive won’t kick in.
About five minutes after Kevin Esvelt invented Crispr gene drives, he freaked out about them. The technology could do plenty of good by preventing the transmission of horrible diseases and controlling animal populations without any killing. But it could also—if used prematurely, greedily, or unilaterally—drive species extinct and destroy public trust in science.
Cerebral, willowy Esvelt is now a professor at MIT and looks as much like an indoor person as Campbell looks like an outdoor one. When asked about the promise and peril of his intellectual creation, he brings up Boo, his rescue cat, who lost the tip of its ear to frostbite before being taken in. He envisions a future when a local gene drive could reduce feral cat populations, much in the way that Campbell wants to reduce rats on islands. “The thought of feral kittens freezing and starving to death is just viscerally painful for me,” he says.

Note that he uses the term “local” gene drive. One of his responses to his freak-out was to come up with ways of containing synthetic gene drives to a set number of generations. He calls one approach a “daisy chain,” which would add a sequence of genetic drivers that must be in place to propel the desired gene change. The first driver in the chain is inherited normally, so when it dies out, the gene drive does too. Tweaking the number of drivers in the chain could theoretically allow you to match the size of the population of creatures you want to get rid of on an island.
This daisy-chain method is still being tested in the lab, and Esvelt feels that, barring attempts to tackle global health crises like malaria, no one should try a gene drive in the wild until there is a proven local drive. This past November, Esvelt cowrote an essay in PLOS Biology in which he responded to New Zealand’s interest in using gene drives to eliminate introduced predators like rats, stoats, and Australian possums. He called the basic version of a gene drive unsuitable for conservation purposes and warned against its cavalier deployment. “Do we want a world in which countries and organizations routinely and unilaterally alter shared ecosystems regardless of the consequences to others?” he wrote.
Esvelt has the same concerns about GBIRd’s early and enthusiastic interest in exploring gene drive technology. GBIRd recently said that its members intend to pursue a “precision drive” approach, in which the drive would work only on animals with a specific genetic sequence—kind of like the fail-safe system Thomas is currently using in the lab, but relying on naturally occurring genes rather than introduced bacterial ones. Researchers would have to locate a DNA sequence found only on the target island and nowhere else, a prospect Esvelt thinks is unlikely. “There is a high chance it won’t work out and they are building up hope,” he says. On larger islands, there would be too many genes coming and going from other places for a perfect sequence.
Although Esvelt supports species conservation, he believes ethical priority must be given to preventing human and animal suffering. “The risk is that you could potentially cause a tragedy in the form of an accidental spread that would delay the introduction of a gene drive to stop malaria,” Esvelt says. “Sorry, I don’t care about endangered species that much.”
But he says he wants GBIRd to carry on—as openly and carefully as possible, and in consultation with the public—because he does care about the suffering of the invasive animals. The poisons that Island Conservation and other environmental groups typically use on rodents cause a horrible death. The rats bleed from internal organs and sometimes their eyes, nose, gums, and other orifices in the course of about six agony-filled days.
Esvelt himself is working on a project to disrupt the cycle of Lyme disease on Nantucket, Massachusetts. The people on the island objected to using a gene drive, so the current plan Esvelt helped develop would simply swamp the local Lyme-susceptible mice with up to 100,000 mice engineered to be Lyme and tick resistant. The hope is that the resistance genes will spread far enough in the population to make a difference. He is willing to let the community set the pace.
Three hundred twenty-five miles north of Thomas’ lab in Adelaide is a remote conservation research station called Arid Recovery, where another experiment to save endangered species is going on—this one with no lab mice at all. It is a forbidding landscape: 30,000 acres of red dunes dotted with tough, thorny scrub and divided into huge fenced enclosures stocked with Australian animals, most of which are on the verge of extinction because they are eaten by human-introduced cats and foxes.
It is so dry in the conservation area that everything left behind simply sits on the sand, seemingly forever, from dead wood to neatly knapped stone tools to the bones of a burrowing bettong (or boodie), something like a cat-sized kangaroo with a huge spherical rump. While the red sand outside the reserve shows prints of rabbits and cats, the dunes inside are inscribed with indigenous tracks: the long heart-shaped back feet of the boodie, the sideways V of the Western barred bandicoot, the distinctive toenail marks of the greater bilby.
Katherine Mosebey, an ecologist who cofounded the reserve, spent years getting rid of the foxes and cats from these fenced areas so the native animals could thrive. Now she is adding a few cats back into some of the swept-clean areas. The idea is to get the boodies and bilbies used to the cats, so that someday they can be released beyond the fence and not be instantly obliterated by predators they do not know how to fear.
The experiment has been running for just a few years, but already the bettongs that have to deal with cats are noticeably more wary. On a starry September night, I went out with the three scientists behind this project: Moseby; Mike Letnic, of the University of New South Wales in Sydney; and Daniel Blumstein, of UCLA. We drove in a Toyota HiLux, and Letnic pointed a bright hand-held spotlight out the window. In the 10-square-mile area with the cats, boodies scampered out of the way of the dusty pickup, their butts like furry bouncing balls. Letnic seemed worried that there were too many cats; the eyes of the feral felines shone in the spotlight, and the night seemed full of them. One agile tabby leaped over a saltbush, disappearing behind a dune. If too many cats reproduce in the enclosure, all the native species will be killed. If t As we passed into the smaller cat-free zone, the boodies seemed noticeably more dim-witted. Several times the truck was forced to stop while someone got out and tried to herd them out of our way. Letnic ran at a couple who gazed at him with mild interest. As he approached, they began running companionably along with him, the man and marsupials looking like three friends out for a jog. In the end, Letnic had to nudge them off the road with the side of his foot. Outside the fence, they would be cat snacks by now.
The difference between these naive animals and the marginally more wary bettongs in the enclosure next door represents learning, but the team is also interested in using the cats as a kind of evolutionary filter. Smarter, faster, bigger, warier bettongs will survive the cats’ wiles and predations, and reproduce. Over the generations, they should become able to coexist with cats.
“It might take 100 years,” Moseby says.
Moseby is working with simple tools—cats, fences, radio collars, and traps—but she’s tentatively interested in the genetic tools on the horizon. A gene drive, if it works, could leapfrog 100 years of learning and evolution and death at the sharp end of a cat’s teeth.

KARL CAMPBELL CAME to the Galapagos as an immigrant and found a home there. He married an Ecuadorian jewelry designer, and they have a daughter. Local people accept him, according to his old boss, Felipe Cruz, formerly deputy executive director of the Charles Darwin Foundation. “People appreciate that he is not one of the passing-by experts.”
Yet his work there hasn’t been without its critics. There were all those dead hawks on Pinzón Island, for instance. Just a dozen of the birds nest there now. But Campbell points out that baby tortoises have been born—the first in more than 150 years—and he counts the effort on the plus side of the ledger. If a small percentage of native animals die, that’s fine with him, because that’s better than 100 percent going extinct.
Campbell insists that he and GBIRd are committed to being careful and deliberate. Pretty much voicing Esvelt’s exact fear, he says, “If you screw it up the first time around, you might put it back 30 years.” In the meantime, he waits and keeps poisoning things, hoping to stave off extinctions and make the islands safe for species that remain.
After visiting the farm on Floreana, Campbell and I had a beer on the beach, watching the sun set. From where we sat, we could see the grave, round heads of sea turtles as they popped above the waves to breathe. Down at the point, sea lions lolled on the sand and crimson Sally Lightfoot crabs scuttled over jet-black lava rocks. The ocean was apricot and silver. Campbell told me that there used to be a crazy-looking turtle genus on Vanuatu—“with a clubbed tail with spikes.” They all went extinct in the first few hundred years after people discovered the island, 3,000 years ago. Humans have been driving things to extinction for a long time. We know how to do that without even thinking. We have less practice dragging them back from the brink.
Originally published by WIRED
Want to learn more?
Check out other journal entries we think you might be interested in.

The Ebiil Society: Champions of Palau
Ann Singeo, founder of our partner organization the Ebiil Society, shares her vision for a thriving Palau and a flourishing world of indigenous science!

Permanent Commission for the South Pacific and Island Conservation Sign MOU to Protect Marine and Coastal Areas in the Southeast Pacific (CPPS)
This historic agreement aims to protect the marine and coastal areas of the Southeast Pacific.

Nukufetau Atoll Bolsters Pacific Conservation and Enhances Community Resilience by Removing Invasive Rats
Our projects to restore key islets in Nukufetau Atoll forecast climate resilience and community benefits in Tuvalu!

New Paper Shows Invasive Species Removal is a Nature-Based Solution for Climate Resilience
Island Conservation and partners have published a new paper quantifying ecosystem resilience on restored islands!
What is Climate Week?
Climate Week NYC: what is it and why is it important? Read on to find out why Island Conservation is attending this amazing event!

The Dynamic Coastlines that Change Island Futures
With sea levels on the rise, how are the coastlines of islands transforming? Read on to find out how dynamic islands really are!
Our Favorite Conservation Photos of 2023
Join us in celebrating the most amazing sights from around the world by checking out these fantastic conservation photos!

Rare Joins the Island-Ocean Connection Challenge
Rare will support the effort to restore island-ocean ecosystems by engaging the Coastal 500 network of local leaders in safeguarding biodiversity (Arlington, VA, USA) Today, international conservation organization Rare announced it has joined the Island-Ocean Connection Challenge (IOCC), a global effort to…

Cryptocurrency Donations: Make an Impact with Your Digital Wallet
Island Conservation accepts cryptocurrency donations. Make an impact using your digital wallet today!

Hope for Globally Threatened Seabirds as BirdLife South Africa Joins Island-Ocean Connection Challenge with Marion Island
For Immediate Release Conservation powerhouse BirdLife South Africa has joined the Island-Ocean Connection Challenge (IOCC) – a global initiative aiming to restore, rewild and protect islands, oceans and communities – to support its work to save internationally significant albatross populations…